The Goicoechea and Aldridge groups have been collaborating on aspects of the chemistry of the Main Group elements for 4-5 years, fostered by the collegial atmosphere in Oxford’s Chemistry Research Laboratory and the physical and intellectual proximity of the two research groups. Even before this, the synthesis and reactivity of unconventional main group compounds was a strong area of interest for both teams: a significant portion of recent work has focused on the design of novel species that challenge conventional paradigms of structure/bonding and reactivity. With this in mind, we set out on a collaborative project to synthesise the first anionic aluminium nucleophile, analogous to the well-known boryl systems pioneered by Makoto Yamashita. Crucial to the success of this project was the involvement of two highly talented researchers - Jamie Hicks and Petra Vasko - who came to Oxford with strong backgrounds in Main Group chemistry.
Trivalent aluminium compounds – as every chemistry student will tell – are electron deficient and act as Lewis acids in their chemical reactions. Numerous synthetic transformations (e.g. Friedel-Crafts alkyl- and acylation) and widely-used industrial processes (Ziegler-Natta alkene polymerization) draw on this characteristic chemical property. Aluminium compounds in the +1 oxidation state are also known, of course - notably the ground-breaking monomeric 'Nacnac' systems developed by Herbert Roesky's group in Göttingen. However, the reverse polarity ‘umpolung’ strategy of using an aluminium reagent as the nucleophilic partner in Al-E bond-forming substitution reactions (E = H, C etc.) has not been experimentally accessible due to the fact that anionic systems of this type have not been available.
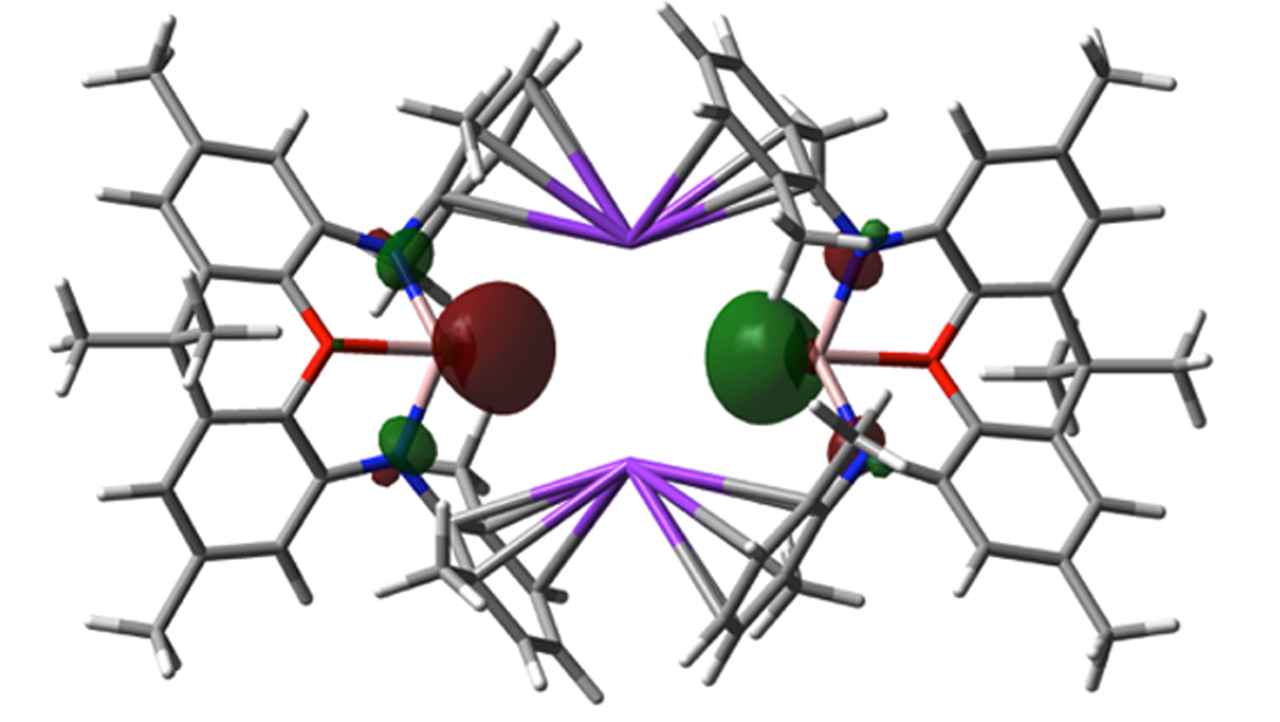
In our report (https://rdcu.be/LGqu), we show for the first time that anionic aluminium(I) compounds can be synthesized, that they can be stable at room temperature, and that they can act as nucleophiles. A dimethylxanthene-stabilized potassium aluminyl compound can be synthesized by potassium reduction of the corresponding iodide, that shows novel modes of reactivity: (i) in ‘umpolung’ fashion as an aluminium-centred nucleophile for the formation of Al-E covalent bonds (E = H, C, M) via substitution chemistry; and (ii) through the formal C-H oxidative addition of benzene - an unprecedented reaction at a single well-defined main group metal centre. As such, we believe that this manuscript describes a step-change in the chemistry accessible to one of the most technologically important elements in the Periodic Table – metaphorically, it turns aluminium chemistry on its head!

The paper in Nature is here: https://go.nature.com/2JSwLew




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in