"Methane is extraordinary", said Prof. Kiyoshi Otsuka when I was doing research on the oxidation of alkanes in my master course. I remembered his words when Prof. Atsushi Fukuoka and I decided to study the oxidation of methane ten years later. Methane is 910,000 times less reactive than n-hexane, a typical alkane, at 298 K, assuming that the C-H bond dissociation energy (439 kJ mol-1 for methane and 405 kJ mol-1 for hexane) linearly correlates with the activation energy of its conversion. In addition, when the C-H bond of methane is cleaved it easily goes to carbon dioxide. We knew these were the challenges ahead of us when we ventured into the chemistry of methane oxidation.
Our initial goal was to look for new catalytic applications of zeolites. The advent of natural gas as an abundant source of methane ensured future interest in the catalytic conversion of methane. At that time, limited reports were available on zeolite-based catalysts for the oxidation of methane to syngas, a mixture of carbon monoxide and hydrogen. It could be the intuition that zeolites are unstable in the presence of water, a by-product of the reaction, at a high reaction temperature of the syngas production, typically 800 °C, which deterred researcher from using zeolites as catalysts for this reaction. Furthermore, the strong acidity of zeolites possibly facilitates coking. Nevertheless, zeolites are structurally ordered and show unique interactions with metal species, which frequently offers unique catalytic sites that cannot be achieved by other supports. Thus, we explored the use of zeolite-supported metal catalysts for the oxidation of methane to syngas. Our primary target was to lower the reaction temperature to 650 °C or less to create a cost-efficient process.
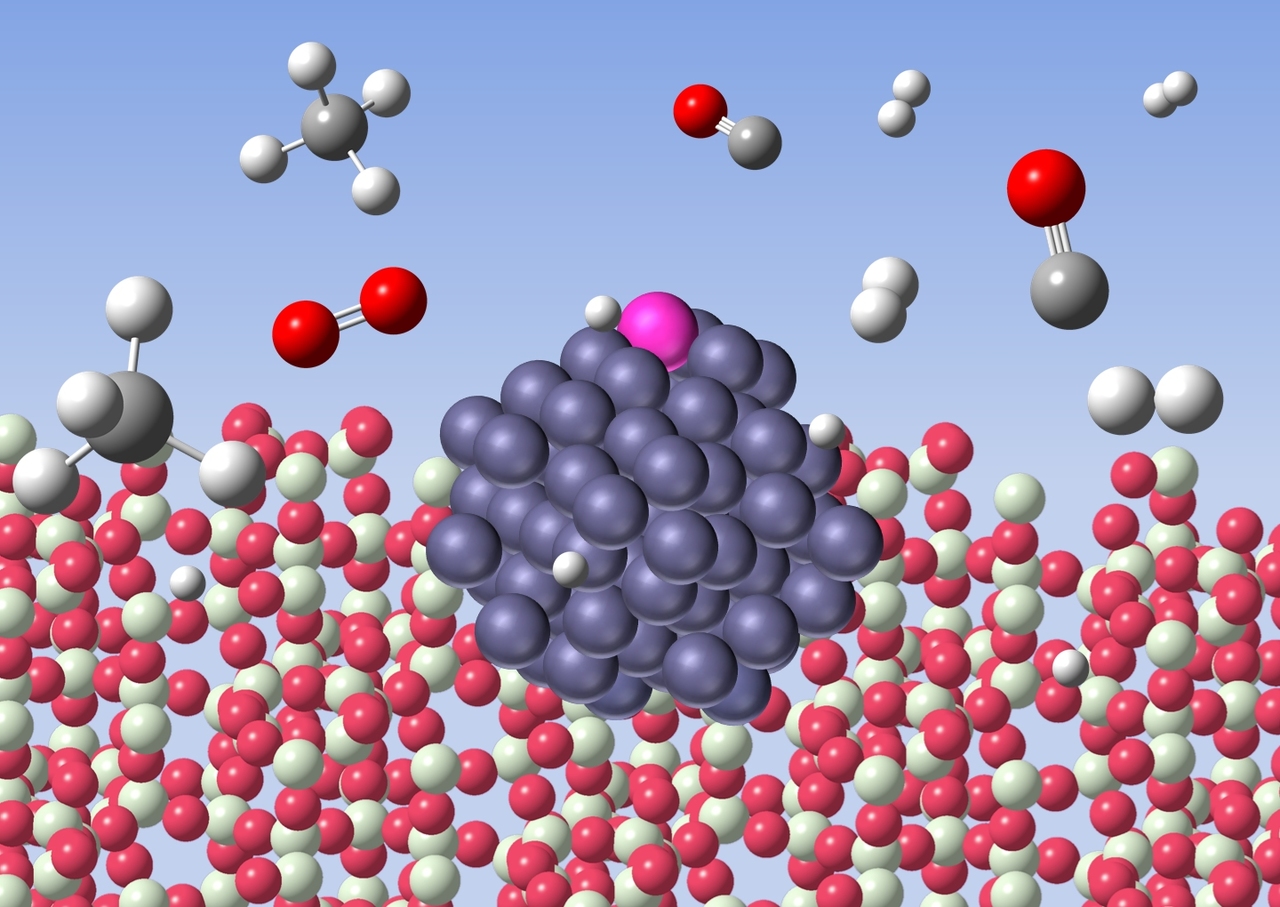
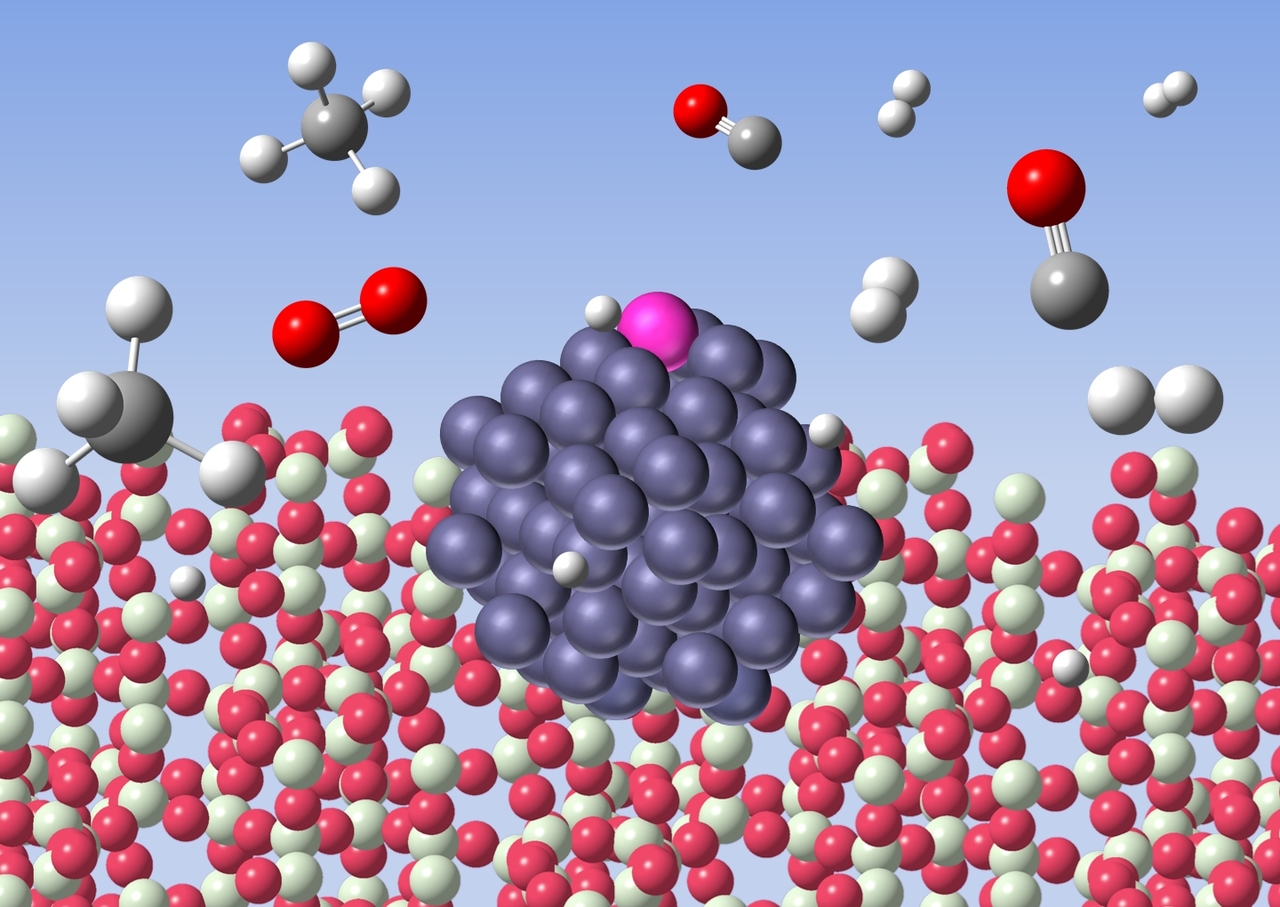
In this work, we developed an inexpensive cobalt-based catalyst for the low-temperature methane oxidation. However, base metals are easily oxidised under the reaction conditions and often cause coking. Here, use of a zeolite support and trace amount of rhodium (0.005%) was the key to overcome these issues. This combination provides atomically dispersed rhodium on the surface of cobalt particles of 1.5 nm average diameter. The rhodium atoms sitting on top of cobalt particles dissociate the methane to produce atomic hydrogen species. These hydrogen species spill over onto the zeolite support and reduce cobalt oxide to zero-valent active species. The small particles of cobalt avoid coking, which is in contrast to larger cobalt particles (ca. 10 nm) that decompose methane to carbon and hydrogen.

Formation of active cobalt species by spillover hydrogen
Preparation of single metal atom sites often requires difficult methods, and zero-valent single metal atoms cause sintering. However, in our method, rhodium is spontaneously dispersed on the surface of cobalt and thus stable. The catalytic property may be tuned by changing the combination of metals and supports. We believe this methodology is useful to design new heterogeneous catalysts for the methane conversion.
This work has been published in Communications Chemistry, see go.nature.com/2vaAi2a



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in