Carbon is one of the most fundamental elements in chemistry. The combination of carbon with metal leads to the development of organometallic chemistry, which vastly accelerates the development of chemistry, biology, and material science. In general, the approach to organometallic complexes should be carried out under inert atmosphere using standard Schlenk techniques or even in gloveboxes. Among the large class of organometallic complexes, there are many attractive metallacycles with unique properties, organometallic reactivity, and aromaticity, which have been regarded as metallaaromatics that greatly expanded the library of known aromatics. The origin of metallaaromatics can be traced back almost 40 years to the first computationally proposed metallabenzene complexes by Thorn and Hoffman15 in 1979 (Nouv. J. Chim. 3, 39-45, 1979), which was synthesised and characterised three years later (J. Chem. Soc., Chem. Commun. 14, 811-813, 1982). I have been fascinated by these distinct organometallic complexes on my doctorate research, and I was lucky enough to discover the first free metallabenzenes with a metal atom from the second transition series, i.e. ruthenabenzenes, just before my graduation (Angew. Chem., Int. Ed. 45, 2920–2923, 2006). Since that time, our interest in these aromatic organometallic complexes continues to this day. A diverse array of monocyclic and polycyclic metallaaromatics has been achieved in our group through the reactions of simple transition metal complexes and substituted alkynes (Wright, L. J. Metallabenzenes: An Expert View (Wiley, Oxford, 2017)).
In our efforts to design metallaaromatic systems, we have been investigating the chemistry of our metallaaromatics, not only with regard to their structure and bonding characteristics but also in terms of their reactivities and physical properties. The metal-carbon bonds have been regarded as the most active reaction sites of metallaaromatics and a variety of related reactions have been demonstrated in the literature. In our investigation, the metal-carbon bonds of metallaaromatics can undergo typical reactions for both aromatic species and metal carbene/carbyne complexes and thereby offer unparalleled scope and versatility, particularly with respect to the stabilization and isolation of some highly labile frameworks (Angew. Chem., Int. Ed. 52, 9251-9255, 2013; Chem. Sci. 7, 1815–1818, 2016; J. Am. Chem. Soc. 139, 1822–1825, 2017). For example, we synthesized the first examples of pentadentate chelates with all binding atoms of the chelating agent being carbon atoms (Sci. Adv. 2, e1601031, 2016), in which the all-carbon-ligating framework has been described as “a cozy carbon warp” for metal center (C&En. 94, 9, 2016).

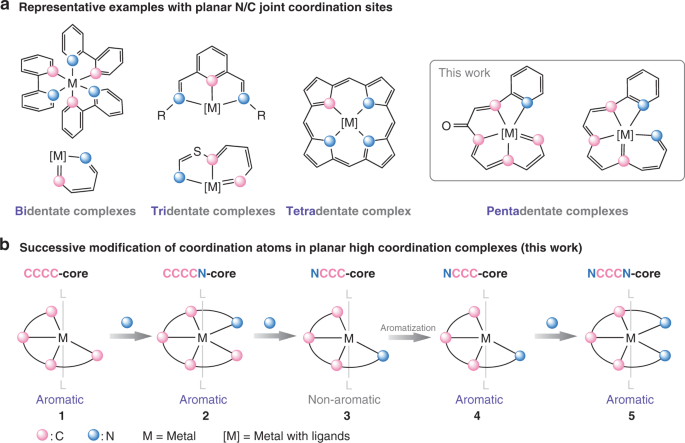
The isolation of the unique pentadentate chelates prompted us to investigate whether unprecedented and exciting N-containing scaffolds exists and, if so, how the synthesis could be achieved. As shown in Fig. 1a, a variety of complexes with N/C joint multidentate ligands have flourished and attracted tremendous research interest because of their fascinating structures and various functions. Generally, these complexes are prepared by aligning N/C joint multidentate ligands with transition metal centers. However, it appears to be unfeasible to synthesize the pentadentate examples involving planar N/C combined ligand systems by common strategies, owing to the inaccessibility of the atypical geometries, especially for the complexes based on transition metal centres.

What can we do to achieve the planar N/C high-coordinate complexes? The first thing that came to my mind was our aromatic complexes with a CCC or CCCC core, which exhibit abundant reactivities either to nucleophiles or electrophiles. We begin to dream of the desired NCCCC pentadentate complex to be obtained by “imbedding one nitrogen atom” to the CCCC core. We were happy to find that metal carbene character and the the strained three-membered metallacyclopropene unit in CCCC complex have done a great favour for the successive and direct transformation of coordinating atoms in complexes. The starting CCCC complex were then converted to the corresponding CCCCN, NCCC, and NCCCN compounds, which could be isolated efficiently under mild condition. Our experimental observations, together with theoretical calculations, reveal the inherent aromatic nature of the complexes with N/C high coordination environment.
The incorporation of nitrogen and carbon atoms into a polydentate ligand framework has been demonstrated to significantly affect the optical and electronic properties. We envisage the synergistic interplay of aromaticity and rich coordination chemistry may result in special properties. Thus, we develop cooperation with Prof. Liu and Prof. Nie to investigate their photophysical properties. The unique planar high coordinated complexes exhibit significant sonodynamic effects and good photoacoustic performances, demonstrating their promise as theranostic agents for PA imaging-guided SDT for cancer or bacterial therapies.
The pentadentate complexes just like newly opened flowers with five petals in “aromatic garden”. We thus get motivated to design other multidentate ligand platform starting from commercially available substrates, even simple metal salt. We anticipate this easy-to-handle method could be applied to synthesize various high-coordinate structures with a variety of metals, which will ultimately be useful for organometallic chemistry and other related fields.

For more details, please see our paper: “Successive modification of polydentate complexes gives access to planar carbon- and nitrogen-based ligands” published in Nature Communications. (https://doi.org/10.1038/s41467-019-09367-8)




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
Very interesting work !