Donor-acceptor Stenhouse adducts (DASAs) are attractive for the reversible visible light/heat-induced linear-cyclic isomerization, which have been widely applied in light-controlled drug delivery, surface wetting and self-assembly. However, since they were firstly synthesized by Read de Alaniz’s group in 2014, DASAs were mostly used as photoresponsive molecules. Read de Alaniz’s group focused on the chemical modification and triggered light shifting of DASAs, while another famous group from Netherland, Feringa’s group focused on the mechanism during light-induced linear-to-cyclic isomerization.
I have been interested in DASAs since I was a Ph.D student in Max-Planck-Institute for Polymer Research in 2015, where I was focused on red-light-responsive supramolecular interactions between azobenzene and cyclodextrin. The host-guest complexes responsive to long-wavelength-light are potential to be applied in biomedical field.
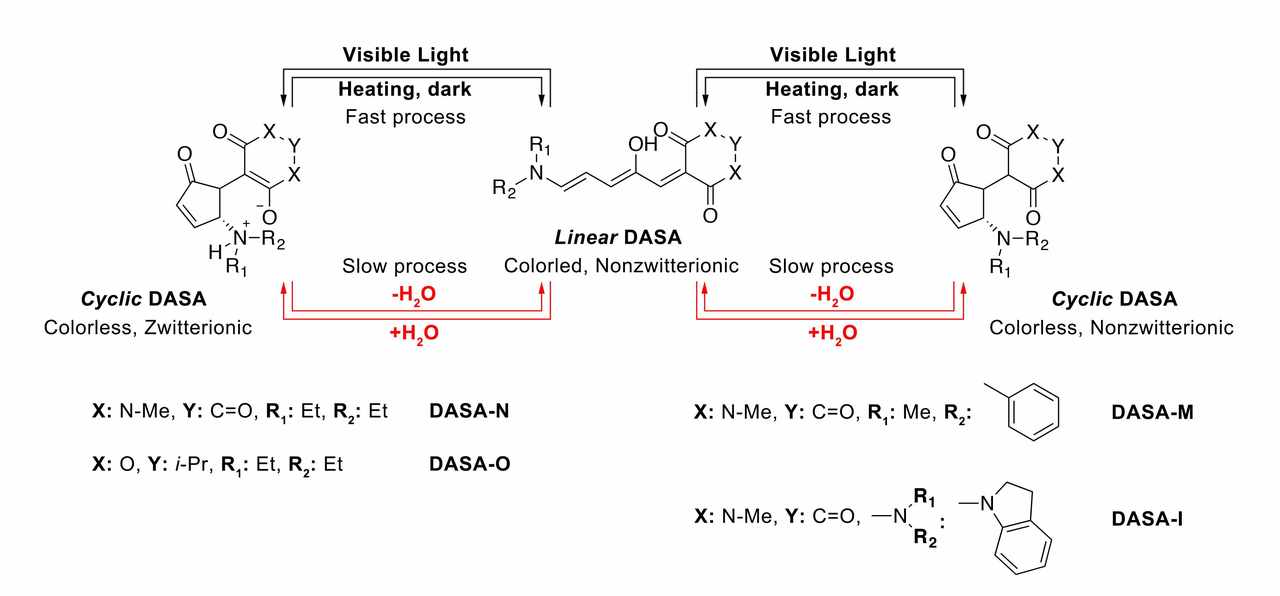
Therefore, after back to China, I decided to work on DASAs. There was one day, I accidentally added one drop of water into the toluene solution of DASA-N, and then lock the mixture into my draw (Figure). Interestingly, I found the purple linear DASA-N solution faded the next morning (Figure). Then we noticed that this phenomenon (water-induced linear-to-cyclic isomerization of DASAs) was also reported by Read de Alaniz’s group and Feringa’s group, however, both of them did not investigate the mechanism and reversibility.

The water-induced linear-to-cyclic isomerization was demonstrated to be a thermal process, that is, cyclic DASAs are stabilized by water molecules. These were further demonstrated by density functional theory (DFT) and molecular dynamic (MD) simulations. DASAs can coordinate with water molecules to form DASAs·xH2O, which further lower the molecular energy of cyclic DASAs. After that we were thinking, is the water-induced linear-to-cyclic isomerization reversible? Then we tried to remove the coordinated water molecules. We tried anhydrous MgSO4 and microwave oven, which did not work. Then after guiding of thermogravimetric analysis, cyclic DASAs·xH2O was heated under 180 oC, which triggered the coordinated water molecules loss and further induced cyclic-to-linear isomerization. This makes DASAs to be applied in information hiding/displaying on paper and color-switching inks.
Therefore, DASAs are not only photoresponsive molecules. This research broadens the investigations and applications of DASAs. It also makes me to rethink the other well-known photoresponsive molecules, including azobenzene, spiropyran et al. Actually, we are still focusing on the mechanism and more applications on the reversible water-induced linear-cyclic isomerization of DASAs. Please keep paying attention to our followed works.
Please read our paper for the full story.
References
- Poelma, S. O. et al. Controlled drug release to cancer cells from modular one-photon visible light-responsive micellar system. Chem. Commun. 52, 10525-10528 (2016).
- Zhao, H. Q. et al. Surface with Reversible Green-light-switched Wettability by Donor-acceptor Stenhouse Adducts. Langmuir 34, 15537-15543 (2018).
- Singh, S. et al. Spatiotemporal photopatterning on polycarbonate surface through visible light responsive polymer bound DASA compounds. ACS Macro Lett. 4, 1273-1277 (2015).
- Helmy, S. et al. Photoswitching using visible light: a new class of organic photochromic molecules. J. Am. Chem. Soc. 136, 8169–8172 (2014).
- Hemmer, J. R. et al. Tunable visible and near infrared photoswitches. J. Am. Chem. Soc. 138, 13960-13966 (2016).
- Lerch, M. M. et al. Unraveling the photoswitching mechanism in donor-accepotor stenhouse adducts. J. Am. Chem. Soc. 138, 6344-6347 (2016).
- Lerch, M. M. et al. Solvent effects on the actinic step of donor-acceptor Stenhouse adduct photoswitching. Angew. Chem. Int. Ed. 57, 8063-8068 (2018).
- Donato, M. D. et al. Shedding light on the photoisomerization pathway of donor-acceptor Stenhouse adducts. J. Am. Chem. Soc. 139, 15596-15599 (2017). Hemmer, J. R. et al. Controlling dark equilibria and enhancing donor-acceptor Stenhouse adduct photoswitching properties through carbon acid design. J. Am. Chem. Soc. 140, 10425–10429 (2018).







Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
Perfect