Electrocatalytic reduction of CO2 with H2O into C2+ compounds is a very attractive but challenging research target. Despite significant progress in this field, high Faradaic efficiency of C2+ products can only be achieved at low current density, limiting the yield of C2+ products. In a recent article published in Nature Catalysis, we present a new strategy to boost CO2 eletroreduction to ethylene and ethanol through hydrogen-assisted C−C coupling. An ultrahigh current density of 1.6 A cm-2 at C2+ (mainly ethylene and ethanol) Faradaic efficiency of 80% could be achieved for electrocatalytic CO2 reduction in a flow cell over a fluorine-modified copper catalyst, which outperforms other reported electrocatalysts. The C2−4 selectivity reaches 85.8% at a single-pass yield of 16.5%, significantly better than those reported for thermocatalytic hydrogenation of CO2 under harsh conditions.
Catalytic transformation of C1 molecules into C2+ compounds via controllable C−C coupling is the core of C1 chemistry. The synthesis of ethylene and ethanol from CO2 is particularly attractive due to their versatility in the chemical and energy industries. Traditionally, the hydrogenation of CO2 with H2 by modified Fischer-Tropsch (FT)-synthesis catalysts could produce C2+ products at high temperatures and pressures. However, the selectivity of a specific C2+ product on the modified FT catalysts is low because of the limitation by Anderson-Schulz-Flory distribution. Moreover, the thermocatalytic hydrogenation of CO2 suffers from the formation of considerable CO and CH4. The activation of stable CO2 molecules under mild conditions and the precise control of C−C coupling of C1 molecules are two of the biggest challenges in chemistry.
Electrocatalytic CO2 reduction reaction (CO2RR) with H2O using renewable electricity offers a promising route for CO2 utilization under ambient conditions. Better control of C2+ products via electrocatalytic CO2RR may be possible because the C−C coupling on electrocatalysts proceeds via mechanism different from that in thermocatalytic reactions. Accompanying with CO2RR, the hydrogen evolution reaction (HER) also occurs as a competitive reaction. The current consensus is that the inhibition of HER is essential to obtain high CO2RR selectivity and C2+ selectivity. However, the catalysts developed based on this consensus are typically less active in spite of high selectivity, and the C2+ yield by electrocatalytic CO2RR is generally lower than those reported for thermocatalytic processes. Therefore, it would be a significant step forward to develop a new methodology to accelerate the activity while keeping the high C2+ selectivity.
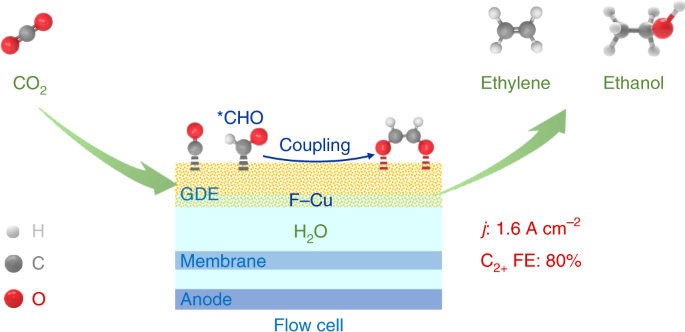
Here, we have succeeded in developing a powerful fluorine-modified copper catalyst for electrocatalytic CO2RR to C2+ products with high selectivity at high activity. An ultrahigh current density of 1.6 A cm-2 was achieved at a C2+ (mainly ethylene and ethanol) Faradaic efficiency of 80% in a flow cell, resulting in a C2+ formation rate of ~4.0 mmol h-1 cm-2, significantly higher than those reported so far. The C2+ selectivity on a molar carbon basis reaches 85.8% (ethylene 65.2%, ethanol 15.0%) with a single-pass C2+ yield of 16.5%. The comparison with thermocatalytic hydrogenation of CO2 to C2-4 products demonstrates that the present electrocatalytic system is significantly more selective for the formation of C2−4 products, in particular ethylene and ethanol, on a comparable or higher level of C2-4 yield.
From DFT calculations and in situ electrochemical attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIRS) measurements, we propose a new hydrogen-assisted C−C coupling mechanism for electrocatalytic CO2RR to C2+ products. In brief, CO2 is first reduced to *CO via *COOH intermediate, and then the hydrogenation of *CO forms *CHO intermediate that subsequently undergoes C−C coupling to *OCHCHO*, leading to the formation of ethylene. The *CO and *CHO species are key reaction intermediates. The activation of H2O to unique H species is an important step. The presence of fluorine on copper surface promotes water dissociation, CO adsorption and the formation of *CHO intermediates.
The present work not only presents a highly active and selective electrocatalytic CO2 reduction catalyst with potential for practical applications, but also offers a new methodology for precisely controlling and promoting the C−C coupling of C1 molecules.
Article link: https://www.nature.com/article...







Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in