Electrochemically mediated carbon capture (EMCC) utilizes electrochemical-swing processes to capture and release carbon dioxide, which is a promising technology to replace conventional methods based on thermal or pressure swings1-3. The mild working conditions allow the system to be operated at ambient temperature and pressure and powered by intermittent renewable energy sources. Besides, the removal of unnecessarily heating integration simplifies the sophisticated design of the system. Therefore, great progress has been made in device engineering and systematic optimization of EMCC in the past two decades.
Redox-active CO2 sorbents play a pivotal role in EMCC as they can either act as pH mediators for indirect CO2 capture in aqueous systems or absorbents for direct CO2 capture in organic solvents. Nevertheless, the practical implementation of EMCC is still hindered by the limited choice of redox-active sorbents, which are overwhelmed by quinoid species4, 5 with only a few exceptions such as bipyridine6, benzyldisulfide7, and phenazine (pH mediator)8. Do not we have any other options for more reliable redox-active CO2 sorbents?
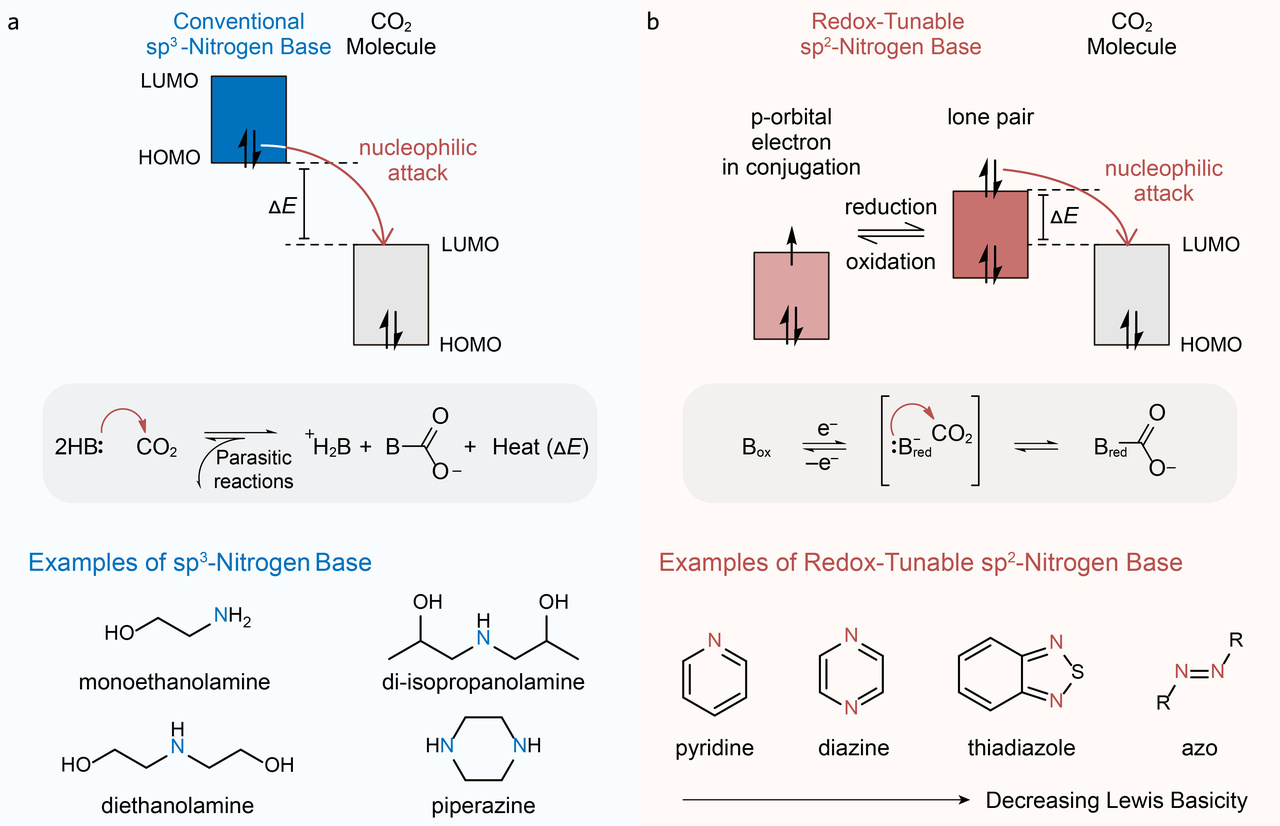
Organic bases with nitrogen centres can be a suitable class of candidates due to their large structural varieties, wide range of basicity, decent solubility and good affinity to carbon dioxide. Conventional sorbents used in amine scrubbing methods9 possess sp3-nitrogen centres, which are not redox-active and form overly stable adducts with CO2 as a result of their relatively strong basicity. As a consequence, enormous thermal energy is required for sorbent regeneration due to the high energy gaps between the highest occupied molecular orbital (HOMO) of amine sorbents and the lowest unoccupied molecular orbital (LUMO) of CO2, often leading to sorbent decomposition and evaporation (Fig. 1). To address this issue, we are motivated to develop a class of redox-active Lewis bases, where their basicity can be tuned by electrochemical potentials. In the neutral state, the HOMO of the basic sorbent sits at a lower energy level than the LUMO of CO2, which favours CO2 dissociation and release; in the reduced state, the energy level of the newly formed HOMO rises, leading to a stronger Lewis base with a moderate binding strength with CO2, which favours CO2 complexation and capture meanwhile ensures a CO2 adduct that is relatively easy to oxidize to the neutral state.
Before the commercialization of the famous “anthraquinone process” for hydrogen peroxide production, both quinone and azobenzene compounds were explored as catalysts10. This intrigued us to think azobenzene or even organic bases with sp2-N centres might exhibit similar redox properties similar to quinone species. Based on this assumption, to our delight, we discovered a big variety of sp2-N bases that can work as redox-active sorbents for EMCC, including pyridines, diazines, thiadiazole, and azo compounds11 (https://www.nature.com/articles/s41560-022-01137-z). Due to the inherent stability of carbamate over carbonate, these sp2-N bases can form CO2 adducts even when they are modified with strong electron-withdrawing groups. Through molecular engineering, we identified two candidate sorbents with more anodic redox potentials than oxygen reduction, 4,4’-azopyridine (AzPy) and 2,7-ditrifluoromethyphenazine. Such property is privileged as it limits the parasitic process in the practical operation of EMCC, where oxygen impurity in the feed gas stream can be reduced to generate peroxide that can oxidize the CO2 sorbent and lower the efficiency of the system. Using AzPy, we demonstrated a flow-based CO2 capture system with promising capacity utilization and release/capture efficiency under simulated flue gas.
Through a combination of experiments and theoretical calculations, our work expands the chemical space and brings insights into the fundamental understanding of electrochemical-swing-controlled CO2 capture and release. The chemical library of redox-tunable Lewis bases can be readily incorporated into a plethora of sorbents in either solid or liquid forms to enhance the intrinsic performance of EMCC. A future aspect of the study can be focused on improving the ionic conductivity of ion exchange membranes in organic electrolytes as well as suppressing the crossover of active species.

Fig. 1 | Conventional sp3 nitrogen bases and redox-tunable sp2 nitrogen bases for carbon capture. a,b, Energy diagrams of the sp3 base–CO2 adduct (a) and redox-tunable sp2 base–CO2 adduct (b), together with the corresponding chemical reactions and molecular examples. The red arrows depict the nucleophilic attack of CO2 by the lone-pair electrons from the bases. B in the chemical reaction equations stands for a base molecule. Box, oxidized base molecule; Bred, reduced base molecule. HOMO, highest occupied molecular orbital.
References
- Stern, M. C. et al. Electrochemically mediated separation for carbon capture. Energy Procedia 4, 860-867 (2011).
- Sharifian, R., Wagterveld, R. M., Digdaya, I. A., Xiang, C. & Vermaas, D. A. Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 14, 781-814 (2021).
- Electrochemical methods for carbon dioxide separations. Nature Reviews Methods Primers 2, 69 (2022).
- Bell, W. L., Miedaner, A., Smart, J. C., DuBois, D. L. & Verostko, C. E. Synthesis and Evaluation of Electroactive CO₂ Carriers. SAE Transactions 97, 544-552 (1988).
- Mizen, M. B. & Wrighton, M. S. Reductive Addition of CO2 to 9,10‐Phenanthrenequinone. Journal of The Electrochemical Society 136, 941-946 (1989).
- Ranjan, R. et al. Reversible Electrochemical Trapping of Carbon Dioxide Using 4,4′-Bipyridine That Does Not Require Thermal Activation. J. Phys. Chem. Lett. 6, 4943-4946 (2015).
- Singh, P. et al. Electrochemical Capture and Release of Carbon Dioxide Using a Disulfide–Thiocarbonate Redox Cycle. J. Am. Chem. Soc. 139, 1033-1036 (2017).
- Jin, S., Wu, M., Gordon, R. G., Aziz, M. J. & Kwabi, D. G. pH swing cycle for CO2 capture electrochemically driven through proton-coupled electron transfer. Energy Environ. Sci. 13, 3706-3722 (2020).
- Rochelle Gary, T. Amine Scrubbing for CO2 Capture. Science 325, 1652-1654 (2009).
- Ranganathan, S. & Sieber, V. Recent Advances in the Direct Synthesis of Hydrogen Peroxide Using Chemical Catalysis—A Review. Catalysts 8, (2018).
- Li, X., Zhao, X., Liu, Y., Hatton, T. A. & Liu, Y. Redox-tunable Lewis bases for electrochemical carbon dioxide capture. Nat. Energy, (2022).




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in