In the late 19th century, a number of factors led to a rapid growth of chemical industry. Petroleum became widely available through new developments in commercial drilling and the demand for synthetic materials to replace their natural counterparts was increasing. After the invention of the first fully synthetic plastic (Bakelite) in 1907, the emergent investigation of new plastics and polymers relied heavily on abundant petroleum feedstocks to provide monomers and precursors. Against this backdrop, industrial chemical production became specialized on petroleum-derived chemicals, a situation which now creates a challenge to overcome with the urgent need to develop new processes that allow us to replace fossil fuel feedstocks with others.
Plant biomass provides an abundant renewable feedstock but the highly oxygenated sugars found in the cell wall can be difficult to transform to the types of highly reduced petrochemicals that are typically used in chemical industry. In contrast, living organisms have evolved over billions of years to utilize sugars for the biological synthesis of small molecule metabolites and macromolecules used by the cell. Indeed, metabolic engineering of microbes has provided a cost-effective way to produce a broad range of chemicals from vitamins to pharmaceuticals. However, pure hydrocarbons with no substituents remain challenging to produce. For example, biodiesel is produced as fatty acid methyl esters and the deoxygenation of fermentation-derived alcohols can be energy intensive. The goal of this work was to develop both a fatty acid synthase (FAS)-independent approach to hydrocarbon synthesis as well as a low energy input method to deoxygenate these molecules to produce hydrocarbons without any substituents.
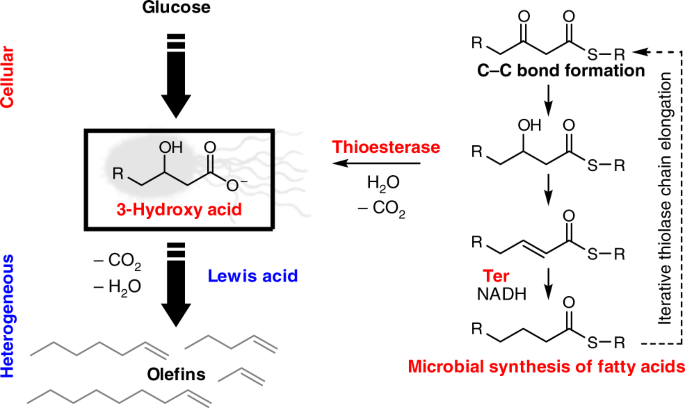
Cells make a large number of hydrocarbons for their cell membranes, but this process is tightly regulated and costs ATP to drive forward the reaction that assembles the monomeric building blocks. We identified new pathways that can produce mid-chain length fatty acids using enzymes orthogonal to FASs, allowing us to optimize production in a genetically-engineered Escherichia coli strain to yield up to 4.3 g/L of the target C8-C10 products. The key to this process was to select an intermediate of hydrocarbon chain growth that contains a 3-hydroxyl group substituent that activates it towards decarboxylation (Figure 1). The product could be extracted from fermentation broth and used directly in the downstream heterogeneous process. A number of zeolite catalysts were found by the Dauenhauer group to be competent to carry out tandem decarboxylation-dehydration by simple Lewis acid catalysis to the (n-1) alkene. We therefore require no redox chemistry to effect the fatty acid deoxygenation, potentially lowering the overall energetic and carbon cost of this process.
This work highlights how combining complementary forms of catalysis - biological and heterogeneous - can lead to new strategies for production of target molecules. In particular, 3-hydroxy acids are readily made via enzymatic pathways and thus accessible directly from sugar. However, further tailoring of the carboxylic acid moiety can be quite challenging given that enzyme selectivity for their native substrates often precludes their acceptance of substrates that are significantly different. By using a downstream heterogeneous process, deoxygenation and conversion to the alkene is relatively agnostic to the R-group structure and opens the door to production of a broad range of olefins using this approach.
You can read more about our work here in Nature Chemistry.

Figure 1. Dual cellular-heterogeneous approach to production of olefins from glucose using acid-base chemistry for deoxygenation. Engineered microbes use glucose to carry out the FAS-independent iterative chain growth of hydrocarbons to produce a 3-hydroxy fatty acid, which is activated for acid-catalyzed decarboxylation-dehydration with Lewis acidic zeolites.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in