It is difficult to overstate the importance of catalysis in society. It is estimated that up to 85% of processes in the chemical industry involve the use of a catalyst.1 Given that the role of a catalyst is, typically, to increase the rate of a specific chemical reaction, thereby increasing process efficiency, they are also seen as an important tool, to tackle the difficult challenges society will face this century and beyond.
The study of heterogeneous catalysis is as fascinating as it is challenging. It is an extremely fluid field of research, which is constantly evolving. Previously, supported bimetallic AuPd catalysts have been demonstrated to be more effective, for a range of different reactions, than the sum of the corresponding monometallic analogues.2 In many cases, this enhancement has been attributed to ‘synergistic effects’; a term that has been frequently employed to describe correlations between the physicochemical properties of the metal nanoparticles and their catalytic performance. Previously, such interactions in bimetallic catalysts have been attributed to electronic, structural and isolation effects. In many cases, the observed synergy is likely to be a combination of these factors but providing conclusive evidence on the nature of the enhancement is exceptionally challenging. In our recent publication in Nature, we report on a new explanation for synergy observed in supported bimetallic AuPd catalysts, using the oxidative dehydrogenation (ODH) of alcohols and formyls as our model reactions.
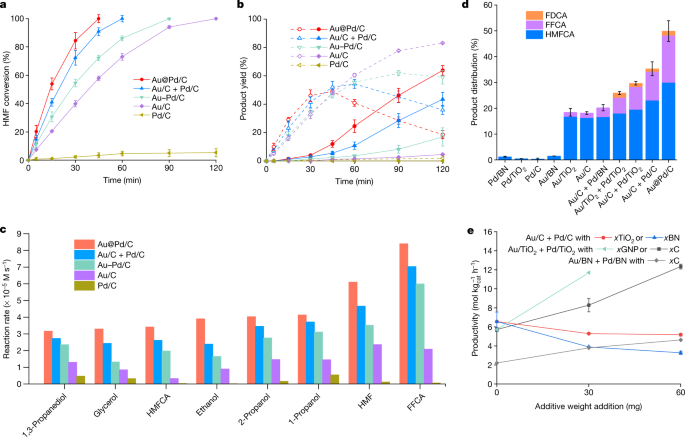
In our work, where we focus primarily on the ODH of 5-hydroxymethylfurfural (HMF) to furandicarboxylic acid, we demonstrate that through elemental separation of Au and Pd nanoparticles, catalytic activity can be significantly improved. This was evidenced by comparing the activities of physical mixtures of Au/C and Pd/C (Au/C+Pd/C) and catalysts that possess spatially separated monometallic particles of Au and Pd on the same support (Au+Pd/C), with conventional bimetallic alloys (Au-Pd/C). Subsequent experiments confirmed that this effect was not limited to the ODH of HMF, demonstrating the generality of the effect (Figure 1).

Figure 1. The spatial separation of Au and Pd in supported bimetallic heterogeneous catalysts enhances catalytic activity in several formyl and alcohol oxidation reactions.
Through combining electrochemical analysis with thermocatalytic testing and materials characterisation, we identified that this enhancement was attributed to the redox coupling of two processes: ODH and oxygen reduction (Figure 2). Electrons generated from ODH on Au surfaces are transferred to separated Pd sites, where they are consumed in an analogous oxygen reduction reaction.3 The discovery that coupling two separate processes in this way can lead to a rate enhancement is a new observation in bimetallic heterogeneous catalysis, which we have termed cooperative redox enhancement (CORE).

Figure 2. Proposed mechanism through which CORE is achieved.
We consider that CORE represents an exciting new frontier in the field of heterogeneous catalysis and provides the community with a deeper understanding of how bimetallic heterogeneous catalysts operate. We hope this work will serve as a foundation for a new research area in catalysis, which will benefit from the collaboration of chemists, physicists and materials scientists.
Find the full paper here: https://doi.org/10.1038/s41586-022-04397-7
References.
- Vogt, C. & Weckhuysen, B.M., The concept of active site in heterogeneous catalysis. Nat. Rev. Chem (2022).
- Kesavan L. et al., Solvent-free oxidation of primary carbon-hydrogen bonds in toluene using Au-Pd nanoparticles. Science, 331, 195-199 (2011).
- Kulkarni, A., Siahrostami, S., Patel, A. & Nørskov, J. K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 118, 2302–2312 (2018).




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in