Carbon dioxide electroreduction (CO2RR) powered by renewable-electricity to chemicals and fuels is a promising route to store the intermittent renewable energy.[1] Renewable methane (CH4) produced from CO2RR, a carbon-neutral alternative to fossil fuels, attracts interest in view of the well-established infrastructure for natural gas storage, distribution, and utilization.[2]
Prior progress in methane selectivity in CO2RR has mainly been made below current density 50 mA cm-2.[3-7] Technoeconomic analyses suggest that practical CO2RR systems require current density above 100 mA cm-2 to make systems profitable.[8] This motivated us to seek to increase, simultaneously, the current density and selectivity of methane production from CO2RR.
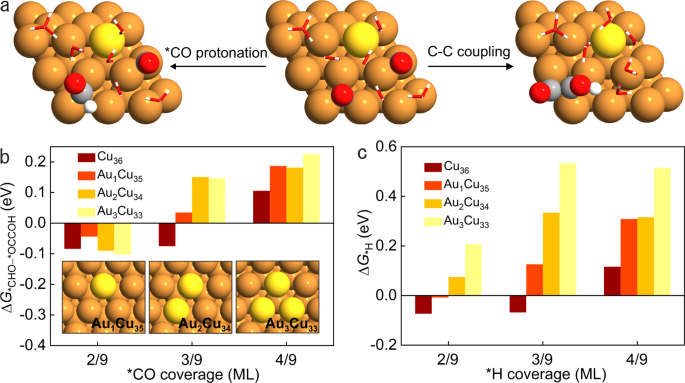
In CO2RR, after the generation of *CO intermediate, *CO protonation to *CHO is the potential-determining step for methane formation, competing with carbon-carbon (C-C) coupling for C2 products as well as the hydrogen evolution reaction (HER).[9-11] Thus, the key to improve methane selectivity is to suppress C-C coupling and HER simultaneously.
In a prior CO2RR study, we found that, on Cu surface, decreasing *CO coverage improved methane selectivity, but with prominent HER.[12] To circumvent the favorable HER and increase the selectivity to methane, we developed a suite of Au-Cu bimetallic catalysts and presented a strategy wherein we controlled *CO availability on Au-Cu catalysts, enabling selectivity to methane at high production rates in CO2RR.
We first fabricated Au-Cu catalysts, based on Cu catalysts supported on polytetrafluoroethylene (PTFE) nanofibers, using a galvanic replacement enabled by the differing reduction potentials of Au and Cu. Using this approach, we obtained a series of Au-Cu catalysts with different atomic percentages of Au in Cu.
In CO2RR, we regulated *CO availability on Au-Cu catalysts through controlling the CO2 concentration and reaction rate. By supplying gas streams consisting of different volume ratios of CO2 to N2, we evaluated CO2RR performance on Au-Cu catalysts under different current densities. Compared to pure CO2, the methane selectivity is promoted on Au-Cu catalysts in CO2–N2 mixed streams at high current densities while the selectivity to ethylene – the main C2 product – decreases dramatically. Relative to Cu catalysts, HER on Au-Cu catalysts is suppressed with increased methane selectivity under low *CO coverage; this leads to a 1.6× improvement in the methane:H2 selectivity ratio compared with prior reports[12-16] having a total current density above 100 mA cm-2. We as a result achieve a methane Faradaic efficiency (FE) of (56 ± 2) % at a partial current density of (112 ± 4) mA cm-2. With the aid of density functional theory calculations and operando X-ray absorption spectroscopy, we found that, under low *CO coverage, the introduction of Au in Cu favors *CO protonation vs. C−C coupling while suppresses HER.
These findings in this work suggest a promising strategy to directly convert dilute CO2 stream to carbon-neutral methane with a combination of high selectivity and high reaction rate.
If you are interested in our work, you may find the full paper here: https://www.nature.com/articles/s41467-021-23699-4

Figure. a, Secondary electron image and the corresponding energy-dispersive X-ray spectroscopy elemental mapping of Au and Cu for the 7% Au-Cu/PTFE catalyst. b, Methane FEs on 7% Au-Cu/PTFE at various CO2 concentrations.
References:
- Jhong, H.-R., Ma, S. & Kenis, P. J. A. Electrochemical conversion of CO2 to useful chemicals: current status, remaining challenges, and future opportunities. Curr. Opin. Chem. Eng. 2, 191-199 (2013).
- Howarth, R. W. & Ingraffea, A. Should fracking stop? Nature 477, 271-275 (2011).
- Qiu, Y.-L. et al. Copper electrode fabricated via pulse electrodeposition: toward high methane selectivity and activity for CO2 ACS Catal. 7, 6302-6310 (2017).
- Manthiram, K., Beberwyck, B. J. & Alivisatos, A. P. Enhanced electrochemical methanation of carbon dioxide with a dispersible nanoscale copper catalyst. J. Am. Chem. Soc. 136, 13319-13325 (2014).
- Li, Y. et al. Structure-sensitive CO2 electroreduction to hydrocarbons on ultrathin 5-fold twinned copper nanowires. Nano Lett. 17, 1312-1317 (2017).
- Reske, R., Mistry, H., Behafarid, F., Cuenya, B. R. & Strasser, P. Particle size effects in the catalytic electroreduction of CO2 on Cu nanoparticles. J. Am. Chem. Soc. 136, 6978-6986 (2014).
- Zhao, H. et al. Computational and experimental demonstrations of one-pot tandem catalysis for electrochemical carbon dioxide reduction to methane. Nat. Commun. 10, 3340 (2019).
- Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165-2177 (2018).
- Peterson, A. A., Abild-Pedersen, F., Studt, F., Rossmeisl, J. & Norskov, J. K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 3, 1311-1315 (2010).
- Cheng, T., Xiao, H. & Goddard, W. A. Free-energy barriers and reaction mechanisms for the electrochemical reduction of CO on the Cu(100) surface, including multiple layers of explicit solvent at pH 0. J. Phys. Chem. Lett. 6, 4767-4773 (2015).
- Gattrel, M., Gupta, N. & Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 594, 1-19 (2006).
- Wang, X. et al. Efficient methane electrosynthesis enabled by tuning local CO2 J. Am. Chem. Soc. 142, 3525-3531(2020).
- Zhuang, T.-T. et al. Steering post-C–C coupling selectivity enables high efficiency electroreduction of carbon dioxide to multi-carbon alcohols. Nat. Catal. 1, 421-428 (2018).
- Ma, S. et al. Electroreduction of carbon dioxide to hydrocarbons using bimetallic Cu–Pd catalysts with different mixing patterns. J. Am. Chem. Soc. 139, 47-50 (2017).
- Jiang, K. et al. Metal ion cycling of Cu foil for selective C–C coupling in electrochemical CO2 Nat. Catal. 1, 111-119 (2018).
- Li, Y. C. et al. Binding site diversity promotes CO2 electroreduction to ethanol. J. Am. Chem. Soc. 141, 8584-8591 (2019).







Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in