To meet the challenge of climate change, new carriers for energy storage have been the subject of intense research, with H2 generated from the splitting of water of particular interest due to its high energy density and carbon-neutral fuel cycle. Energy storage is also an age-old phenomenon in biological systems, where N-heterocyclic pyridine rings (such as in the critical energy molecule NADH) are reduced by hydride transfer into high-energy forms so that the energy can be used in downstream biocatalytic reactions. As interest grows in the utilization of biocatalytic reactions to access molecules that are difficult to prepare, attention has turned towards the regeneration of NADH by artificial, non-enzymatic means.
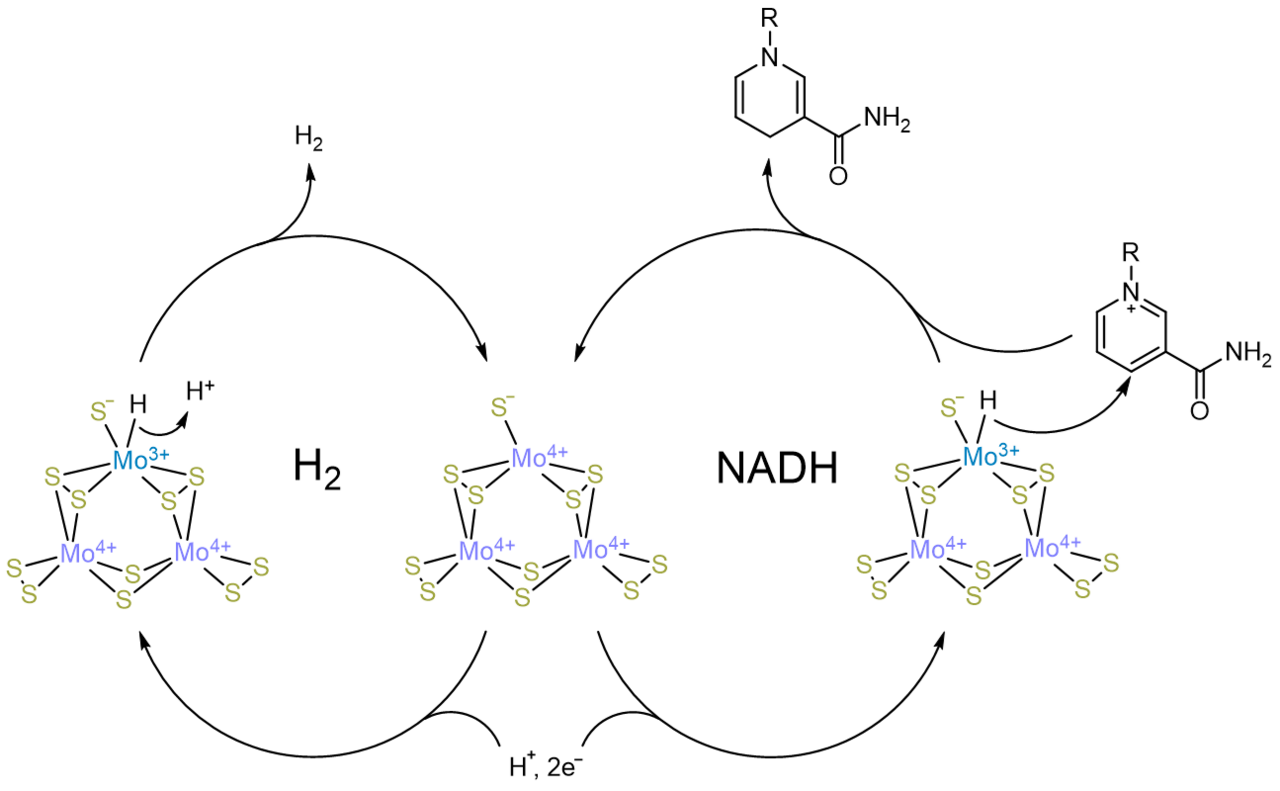
In our work, we demonstrate that the earth-abundant H2 evolution catalyst molybdenum sulfide works for selective NADH regeneration for the same reason as it functions for H2 evolution - through the formation of metal hydride species in water under reducing potentials. This finding not only demonstrates that these catalysts form H2 through a metal hydride intermediate, but also opens the road to cheap, effective NADH regeneration for biocatalysis.
Fundamental evidence for a metal hydride in H2 evolution...
Intrigued by the presence of Mo in numerous H2 evolving catalysts that have been recently reported, we decided to examine Mo sulfide for the presence of Mo3+, an oxidation state that has been associated with H2 evolution activity, using electron paramagnetic resonance spectroscopy (EPR). Not only did the Mo sulfide EPR spectrum exhibit the Mo3+ peak (depending on the deposition condition), it was significantly wider than previously reported peaks. Our analysis of the spectrum led us to confirm that this widening was characteristic of the presence of a Mo3+ hydride. This discovery therefore was direct evidence that Mo is the active site.
...has direct implications in biocatalysis
To confirm the chemical reactivity of the hydride, we used N-methyl nicotinamide (NMN), a simpler analog of NADH, as a hydride scavenger, finding that Mo sulfide could indeed hydrogenate NMN. However, unlike conducting electrodes such as glassy carbon, Mo sulfide transferred hydride specifically without transferring electrons. This result further drove us to see if we could use Mo sulfide for the regeneration of NADH. Specific NADH regeneration is a challenge in the biocatalysis field; many conventional electrocatalysts transfer single electrons when used for NAD reduction to NADH, leading to breakdown products that are not bioactive. Our test system - alcohol dehydrogenase for benzaldehyde to benzyl alcohol - functioned when "powered" by a Mo sulfide electrocatalyst.
Read more about our work in Nature Catalysis: https://www.nature.com/articles/s41929-022-00781-8






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in