What are the chances?
You can often talk yourself out of doing reactions; thinking that they won’t work or that they would be a waste of resources. Laboratory hours are only finite and there are always more important things to be focussing on. Sometimes however, you should just give it a go on a Friday afternoon.
There is an apocryphal tale about the industrial sector during the booming years of the 20th century. Research scientists within some of the largest manufacturing companies were allegedly given carte blanche at the end of the week to be creative. Using their experience and expertise, technicians could explore interesting side reactions, examine strange precipitates and experiment with new ideas to see what sticks. From this, a myriad of commercial products were discovered including, somewhat ironically, Teflon. Academia has also been known to ‘go rogue’ just before a weekend. Nobel Prize winners, Professor Andrei Geim and Professor Kostya Novoselov, frequently conducted ‘Friday night experiments’ and serendipitously discovered a simple method of isolating graphene sheets using sticky tape.
For this paper, that ‘give it a go’ moment was Friday 13th July 2018.
Pumps, past and present
For background, biomolecular pumps and motors are prevalent throughout biology due to their ability to catalyse the hydrolysis of chemical fuels, such as adenosine triphosphate, and use the energy released to direct motion through information ratchet mechanisms. This is crucial for a variety of metabolic processes. Understanding, designing and optimising efficient artificial analogues has been a long-term goal for many research groups around the world.
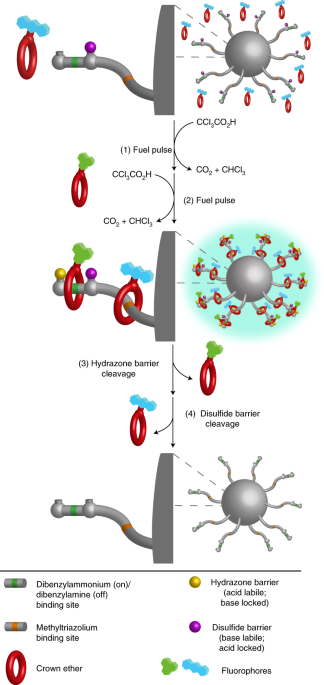
In 2017, the Leigh Group described chemically driven artificial rotary and linear molecular motors that operate through a fundamentally different type of mechanism (Figure 1). The directional transport of substrates away from equilibrium by linear molecular pump 1 is induced by acid-base oscillations. The changes simultaneously switch the binding site affinities and the labilities of barriers on the track, creating an energy ratchet. The linear molecular motor is driven by aliquots of trichloroacetic acid (CCl3CO2H), a chemical fuel that undergoes base-promoted decarboxylation, generating relatively innocuous chloroform (CHCl3) and carbon dioxide (CO2) as the only waste products of machine operation. The system uses the change in relative binding affinity of macrocycles for different binding sites on linear tracks and a gating system based on dynamic covalent chemistry, both of which are affected by switching between acidic and basic conditions. The combination of these processes causes the generation of out-of-equilibrium concentrations of a substrate using a linear molecular pump.

Reinventing the pump
Moving from that first design, the size of the macrocycle was reduced from dibenzo-30-crown-8 (DB30C10) to dibenzo-24-crown-8 (DB24C8), which substantially increased the binding affinity between the crown ether and the dibenzylammonium groups. Consequently, the pump’s threading efficiency was improved which permitted in situ analysis of the operation. By utilising a smaller macrocycle however, several changes to the steric bulk of track were required before the crown ether could shuttle along. As such, the new pump design, 4, removed the terminal adamantyl group and reduced the size of the internal disulfide barrier whilst retaining the previously established binding sites (Figure 2).

Figure 2 – Optimisation and application of Leigh’s 2017 artificial molecular pump.
With a second-generation design in hand, we looked towards the broader applications of these molecular machines. One such proposal was to combine solution-phase artificial molecular pumps with a solid-phase polymer support, forming a phase-transfer pump (6). The sorption of species from solution into and onto solids underpins the sequestering of waste and pollutants, precious metal recovery, heterogeneous catalysis, analysis and separation science, and other technologies. The transfer between phases tends to proceed spontaneously, in the direction of equilibrium. Molecular ratchet mechanisms, where kinetic gating inhibits or accelerates particular steps, makes it possible to progressively drive dynamic systems away from equilibrium.
To investigate the feasibility of phase-transfer pumping, we synthesised model polymer-bound pump 7 (Figure 3). Traditional methods of analysing solution-phase chemistry (nuclear magnetic resonance spectroscopy, mass spectrometry, chromatography etc.) are less insightful or no longer applicable when working in the solid-state. To monitor the operation of this pump, we decided to utilise fluorescence, which is easily analysed with both fluorescent microscopy and spectroscopy. We considered a series of fluorophores and settled on the naphthalimide moiety to conduct preliminary experiments, owing to its ease of synthesis and strong fluorescent response.
Give it a go!
On Friday 13th July 2018, with both naphthalimide-tagged DB24C8 5a and polymer-bound model pump 7 in hand, we gave it a go. Mixing the compounds together in acetonitrile with CF3CO2H for two hours, followed by a series of washes and filtrations to remove any unbound fluorescent macrocycles, afforded 8 (Figure 3).

To our amazement, the fluorescent intensity of the polymer beads was visible to the naked eye! We joined the #FluorescentFriday Twitter community and spent the afternoon excitedly repeating reactions, checking variables and iterating the purification procedure to be confident of what we were observing. Handling the polymer beads was the steepest learning curve. Static electricity (gloves), shaky hands (Dr Daniel Tetlow) and nearby air currents were all strictly forbidden during operations.
By collaborating with colleagues at The University of Manchester (Prof. Simon Webb and Dr Derren Heyes), we were able to use both fluorescence microscopy and spectroscopy to gain valuable insights into our system. After all was said and done, we had demonstrated a model system that could load and unload fluorescent cargo.
Head towards the light
Considering the success of this proof-of-concept study, our attention turned to the phase-transfer pump 6. With two binding sites present, two distinct fluorophores could be incorporated to demonstrate the inherent sequence specificity of the system. Utilising facile amide couplings between the macrocycle and cargo, we searched for alternate fluorescent tags (Figure 5).

Some fluorophores were too reactive, some were not responsive enough and some were unfortunately incompatible with our previously established operation conditions. Thankfully however, the anthracene moiety was found to be the ‘goldilocks’ fluorophore and most suitable for our system.
Just add fuel
In the subsequent months, we synthesised the phase-transfer pump 9 and repeated our experiments, finding parity with the original model 7 and the solution-phase analogue 4. Finally, by modulating the operation with CCl3CO2H, we afforded the sequential pumping of cargo from solution onto polymer beads with a pulsed chemical fuel (Figure 6). The rest of the story can be found online.

It can be said that the best time to tackle a challenge, aside from on a Friday afternoon, is when the surrounding technology has developed sufficiently to solve the problem. From its inception in the mid-20th century, the field of supramolecular chemistry has continuously pushed the limits of what could be achieved using contemporary technologies.
Pioneering reports of interlocked structures in the 1960s, specifically the first [2]rotaxane by Harrison and Harrison, utilised the repeated statistical threading and capping of polymer-bound macrocycles. In the following sixty years, methods of synthesising and analysing the formation of interlocked molecules have developed to a remarkable extent. As such, we were able to demonstrate the non-equilibrium sorption by immobilised artificial molecular machines, which utilised an energy ratchet mechanism to form polymer-bound [3]rotaxanes. This enabled the transduction of energy from chemical fuels for the storage and release of energy and information.








Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in