Alkenes are fundamental chemicals in industry for the large-scale production of a wide variety of products such as detergents, polymers, soaps, lubricants, cosmetics and fragrances.[1] However, internal alkenes are generally much more expensive than their corresponding terminal alkenes, which limits their use and subsequent transformations. The simplest way to obtain internal alkenes is through isomerization reactions of their terminal analogs, which simply consist of the migration of the double bond along the chain to the desired position.[2] However, the reported methods for this reaction generally use metal loadings, ligands, additional additives or solvents, which makes them neither environmentally friendly nor industrially viable.[3-6] In addition, branched and/or oligomerized alkene products are usually obtained, which are unacceptable for many applications.[7] Therefore, the search for a new isomerization methodology applicable to a wide range of organic molecules, is still of high interest. Figure 1 shows different methodologies and drawbacks to carry out the isomerization reaction in industry and academia.

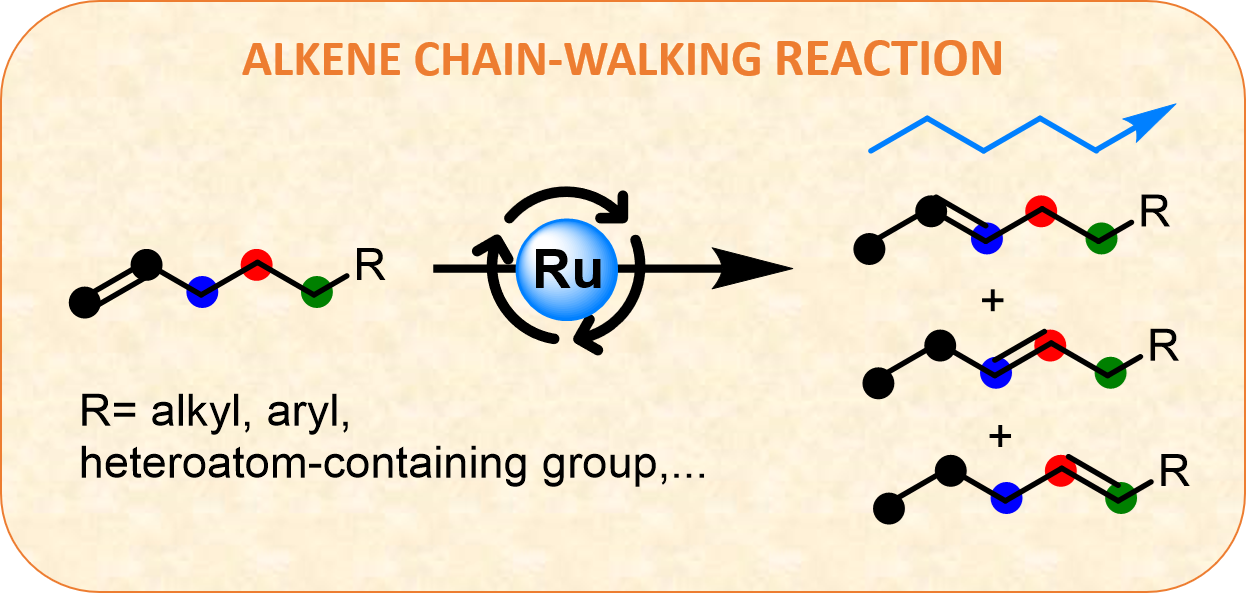
In order to study the reaction, we choose methyl eugenol as a model substrate because it is found in different essential oils and is often used in perfumes or as a flavoring agent. First, we tested different salts and metal complexes as catalysts at 150 ºC under solventless conditions, observing quantitative conversions only with Ru. Therefore, we focus on the use of a wide variety of Ru compounds. To our surprise, almost all of them gave good yields at only 10 ppm, showing 5-20 min induction time. This led us to think that the same Ru active species were formed under solvent-free heating conditions regardless of the Ru source. Since ultra-small amounts of catalyst are used, it is difficult to know exactly what kind of active Ru species are formed in-situ during the reaction. However, we are very curious about this, so we continue to work on it.
In addition, we tried to do the reaction at different temperatures, and increasing the temperature the conversion was greater at the same reaction time. These results suggest that the catalytically active species are stable under heating conditions and that the activity can be increased with the reaction temperature, the only limitation is the boiling point or decomposition temperature of the neat substrate. In order to assess the scope of this methodology, several terminal alkenes containing different functional groups were isomerized with ultralow amounts of Ru, even carbonyl groups have been synthesized from alcohols by migration of the double bonds, which avoids the use of expensive and/or toxic oxidizers to get them. Nevertheless, internal or germinal alkenes are unreactive under these conditions, being a regioselective process.
With these results in our hands, and on the basis of the collaboration that our group has with the company International Flavors & Fragrances Inc. (IFF), we tried to implement the technology developed to obtain two IFF commercial fragrances, known as VeraspiceTM and IsorosalvaTM, which we achieved using parts–per–million of Ru at kilogram scale. These exciting results have made it possible to improve the current production processes for these fragrances, since the production costs are drastically reduced under our conditions. In addition, the fact that ppm of catalyst are used, keeps the metal amount below the legal trace limits and it does not require any additional separation step after the isomerization reaction, which enhances the feasibility of the process on an industrial scale. On the other hand, our procedure also enables that the resulting internal alkenes are ready to be used in different organic reactions without any further treatment.
We want to emphasize that the synthesis of fragrance compounds by the Ru–catalyzed process has been presented to be protected, Patent EP21382234.
Do you want to know more details? Take a look at our article published in Nature Communications: https://www.nature.com/articles/s41467-022-30320-9
References
[1] Keim, W. Oligomerization of Ethylene to α–Olefins: Discovery and Development of the Shell Higher Olefin Process (SHOP). Angew. Chem. Int. Ed. 52, 12492−12496 (2013).
[2] Hilt, G. Double Bond Isomerisation and Migration—New Playgrounds for Transition Metal–Catalysis. ChemCatChem 6, 2484–2485 (2014).
[3] Kapat, A., Sperger, T., Guven, S. & Schoenebeck, F. E–Olefins through intramolecular radical relocation. Science 363, 391–396 (2019).
[4] Z. Lv, Z.et al. A General Strategy for Open–Flask Alkene Isomerization by Ruthenium Hydride Complexes with Non–Redox Metal Salts. ChemCatChem 9, 3849–3859 (2017).
[5] Zhuo, L.–G., Yao, Z.–K. & Yu, Z.–X. Synthesis of Z–Alkenes from Rh(I)–Catalyzed Olefin Isomerization of β,γ–Unsaturated Ketones. Org. Lett. 15, 4634–4637 (2013).
[6] Woof, C. R., Durand, D. J., Fey, N., Richards, E. & Webster, R. L. Iron Catalyzed Double Bond Isomerization: Evidence for an FeI/FeIII Catalytic Cycle. Chem. Eur. J. 27, 5972–5977 (2021).
[7] Basbug Alhan, H. E., Jones, G. R. & Harth, E. Branching Regulation in Olefin Polymerization via Lewis Acid Triggered Isomerization of Monomers. Angew. Chem. Int. Ed. 59, 4743–4749 (2020).






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in